Green Button
The Clinical Informatics Consult Service Thousands of Clinicians and Researchers Use, and Love. Get On-Demand Evidence in 48 Hours Simply by Asking a Question. Every Study is Clinician-QC’d.
Publication-Grade Evidence for Every Clinical Question
Submit a question. Atropos does the rest. Receive a novel retrospective observational study in 48 hours. Learn and ask again.
It’s really that simple. Get Proof.
What’s Your Question?
Just specify the Population, Intervention, Comparison, Outcome, and Timeframe (PICOT)
What are outcomes and subsequent therapies for melanoma patients who received adjuvant PD1 inhibitors?
In all cholesteatoma patients (regardless of congenital or acquired) what are the incidences of risk factors, procedures, and complications?
How many patients coming for major surgery at my hospital had a preop ICD10 code related to depression?
For patients with Parkinson's disease that require pharmacotherapy, how do clinical endpoints vary per classes of FDA-approved therapy?
What is the unmet need in multiple myeloma?
For patients with X condition, X comorbidities, X demographic, X age, what is the difference in outcomes between treatment A and treatment B?
From PICOT to Prognostogram
Atropos produces publication-grade observational study reports to answer your questions in 48 hours. This is achieved with automated retrospective observational studies — all of which are reviewed by Atropos clinicians with informatics expertise.
Expert Intake
Meet Atropos’ Clinical Informaticists
Clinicians in the loop help you generate questions, choose the right data, translate from queries to phenotypes, and QC findings.
Rapid Search
Temporal querying, advanced cohorting
With a temporal query language faster than SQL and a powerful search engine, Atropos can derive custom cohorts from clinical data in seconds.
Automated Analyses
Explore the technology
Templated study designs and a pipeline of automated analyses delivers methodological consistency and accuracy. Tech features machine learning, advanced statistics, and continual refinement.
Publication-grade Reports
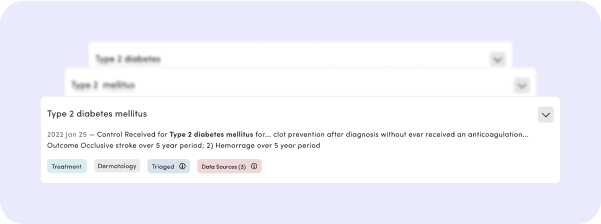
See a Prognostogram
Receive a quick summary and conclusions backed by a detailed epidemiological report in two days. Accessible in your account and the library.
Using the Green Button Informatics Consult Service
The evidence available today is no longer the limit for evidence-based practice.
Use the Green Button to close every evidence gap you encounter in research, practice, or curiosity.
Access Evidence With:
Speed
From question to RWE in 48 hours
Rapid temporal query language
Low code medical data search engine
Custom cohorting
Automated Statistical templates and pipeline
Ease
Submit questions in conversational format
Quick and easy iteration
Simple UI for requesting, reviewing, and sharing information
Atropos clinician-in-the-loop evaluates every result
Breadth
Works across clinical specialties and treatment areas
Can operate on your data, securely within your environment
Access insights from a vast network of medical data, ranging from broad and national to specialty (oncology, dermatology, and more)
Thousands of questions answered, trials re-run, and hypotheses tested
Rigor
Consistent, peer-reviewed methods produce trustworthy results
Clinician’s oversee production and quality of results
Suite of automated analysis features advanced statistics
Technology refined over 12+ years of study and testing
The Impact of Evidence in Action
Patient
-
Patients who don’t match the cohorts studied in RCTs or documented in existing literature often have needs that are unmet by existing standards of care. Healthcare outcomes are often worse for patients like these. Physicians who encounter these and other complex cases may have difficulty managing them.
-
Any patient with a rare disease, multiple comorbid conditions, who is elderly, pregnant, a woman, or in another group commonly underrepresented in trials can finally benefit from personalized care. Earlier diagnoses, interventions, and tailored treatment plans can reduce suffering and save lives.
Groups
-
As certain groups of patients consistently fall through the evidence gap, healthcare disparities perpetuate. From life science companies to insurance plans and healthcare organizations, attempts to resolve these inequities are stymied by seemingly insurmountable barriers.
-
Care protocols for diverse groups are refined over time, as the body of novel observational evidence grows. Accessible evidence also makes jobs easier - for data scientists working with RWD, physicians freed to operate at the top of their licenses, and scientists for whom discoveries are catalyzed.
Institutions
-
Significant IT infrastructure investments can seem like sunk costs as clinically-relevant information remains locked in the EHR system. Targeting population health, quality, or research initiatives is inherently limited by analytics support time and other resource constraints.
-
Healthcare organizations experience the clinical, fiscal, and administrative benefit of fully utilizing their medical data. From bench to bedside, see the benefits of rapid evidence and analytics. Population health and quality improvement initiatives ramp in pace and progress.
Science & Medical Fields
-
It takes months to produce abstracts and posters, and nearly a year for manuscripts. Limits on investigator time, analytics support, data access, or breakdown in communication across cross-functional teams stall scientific discovery. Only the few lines of inquiry prioritized are addressed.
-
Research acceleration and enablement of evidence-based practice set new standards for care, and unlock new avenues for scientific discovery. Answers to previously unanswerable questions become available at the point of curiosity.